
1. How did the idea for the TBSend card come about? What inspired it?
In 2013, Wobble Base, started by an experienced molecular microbiologist, chose tuberculosis (TB) as its primary focus due to its alarming statistics in India and worldwide. Concurrently, a project on developing HIV-1 drug resistance assays using PCR-sequencing was underway, during which TB emerged as a significant opportunistic infection. Weekly lab meetings highlighted the severe impact of TB on the population and the government’s financial burden in combating the disease. For us, it became clear that TB diagnostics needed to be an immediate priority.
Initially, the focus was on TB detection through genes, targets, primers, platforms, dyes, labels, and related research. However, as the project progressed, the team realized that the real challenge was in obtaining and storing appropriate samples. Sputum samples from distant locations often faced contamination, damage to containers, and the dependency on cold chains, compounded by the limited storage capacity of the lab’s small refrigerator which often resulted in poor quality samples, which had the potential to impact the results of the subsequent confirmatory tests.
With time it was realized that it was comparatively easy to design PCR assays and use software to design primers and probes in the lab. But all of these have limited use unless there is a robust solution that ensures a sustained flow of quality samples. Gradually, all staff started brainstorming on how to create a novel solution where samples can be stored when sample flow is good and can be used when their availability is scarce.
Incidentally, our then lab manager, emphasized the need to conserve refrigerator space and offered wooden cupboards for storage, which further fuelled our creativity towards the creation of the TBSend card innovation. After multiple discussions and collaborative efforts of our team for over four long years, the TBSend Card was born.

2. What is unique about the TBSend card and what can it be used for beyond TB?
The TBSend card is a simple yet unique device designed for the long-term storage and transportation of sputum samples. It has been shown to preserve the DNA present in the sample for over six and a half years. When needed, the DNA can be easily released by dipping the card into a one-step DNA release buffer, making it ready for loading onto an Xpert cartridge that is used for further confirmatory testing.
The released DNA’s purity can be further enhanced through a straightforward, one-step centrifugation process that takes just 2-5 minutes. The TBSend card also features technology that enables sophisticated, unbiased whole genome amplification with just 10 minutes of isothermal incubation, significantly boosting its sensitivity. Storage of these cards is hassle-free; they can be kept in a lab cupboard without concerns about fungal growth, contaminant proliferation, or DNA degradation. The DNA remains intact on the IP-protected cellulose mesh until it is extracted.
The TBSend card is compatible with TB CB-NAAT and all forms of TB-NAAT. Moreover, it works beyond sputum and can be used with any of the body fluid samples, making it ideal for storing critical plasma samples for later PCR testing, such as for Hepatitis B virus. The initial pilot biosafety assessments of the TB Send Card have demonstrated favourable results.
3. What stage of development and deployment has it reached?
The TBSend card has reached Technology Readiness Level (TRL) five, i.e. it is being validated or demonstrated in a relevant lab/clinical environment. As a “breadboard technology,” it is set to undergo more rigorous testing in realistic conditions. Upon successful testing, it will advance to TRL 6.
4. How does it support better diagnostics and how easy is its adoption?
Current TB-NAAT solutions often rely on standalone approaches for initial sample handling. After collecting sputum in a container, senders must decide how to send it, ideally following prescribed protocols. If not processed immediately, sputum requires cold chain facilities. DNA extraction is critical for CB-NAAT, with separate modules for LPA, but other PCR-based assays leave extraction to the operator. One needs to choose a third-party extraction kit or find a home-grown method to extract it.
In this context, the TBSend card module addresses the entire sample handling pipeline. It still uses the same procedure for sputum collection that has been in use since the late 19th and early 20th century. However, immediately thereafter, the TBSend card protocol takes over. Sputum is mixed with a buffer and spotted onto the card, making it ready for room temperature transport and storage. DNA extraction is integrated: a one-step DNA release buffer for CB-NAAT or a brief centrifuge for other NAATs is to be used. Further, a sophisticated but simple-to-use, 10-minute whole genome amplification system is integrated in the module that enhances sensitivity for all forms of NAAT with special reference to LPA. This consistent protocol, from spotting to DNA release, eliminates variation.
The TBSend card eliminates the dependency on cold chains and its associated costs and allows for easy storage which is a critical supply chain component. It can transport, and release DNA from various body fluids, functioning as a nucleic acid hard disk. It even works with paraffin-embedded tissue samples after processing. The card ensures superior diagnostic performance through uniform protocols, easy handling, and DNA integrity.
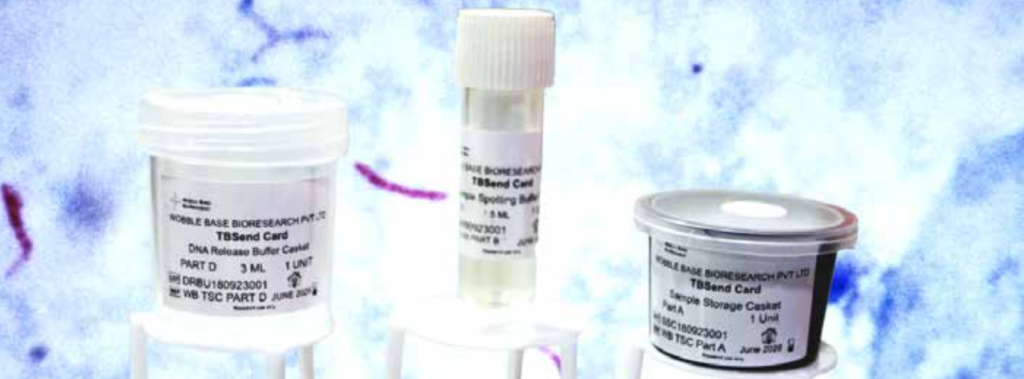
Adoption of a TBSend card is easy since all it requires is to add the body fluid to a spotting buffer and spot on the card. Transportation and storage are all at room temperature requiring no special space or protocol. Further, release of DNA is simple; For CB-NAAT, it is just immersing in a DNA release buffer and for other NAAT tests, arranging for a brief 2–5-minute spin. Whole genome amplification is a simple 10-minute incubation in a simple water bath wherein an ultra-complex reaction takes place in the tube by mere addition of a reaction mix and the process amplifies the entire genome multiple fold in a matter of minutes. This adds sensitivity to the NAAT protocol that is to be followed by making more templates available for detection. Moreover, this innovation is compatible with all NAAT and CB NAAT platforms which addresses one of the critical areas of sample storage and transport within the national TB program.
5. Any first responses from healthcare providers on TBSend Card?
“We commend the innovative design and efficiency of the TBSend card module, which streamlines the process of sputum handling for both healthcare providers and patients, ensuring accurate diagnostic results and satisfactory preservation of samples. This device represents a significant advancement in the commitment to deliver high-quality archived DNA by prioritizing convenience and storage of sputum transportation logistics and bringing about its seamless integration with CB-NAAT as seen with our Cepheid GeneXpert MTB/RIF system platform.”
Dr Dhanji P Rajani
Consultant Medical Microbiologist
Microcare Laboratory & Tuberculosis Research Center, Surat, India ·
Accredited by National Accreditation Board for Testing and Calibration Laboratories (NABL) and Central TB Division, Ministry of Health, Government of India.
6. How does a TBSend card fit into the overall TB diagnostic pathway and what hurdles remain for its seamless integration?
The TBSend card streamlines the initial stages of sample logistics, including collection, transportation, storage, and DNA release. It offers economical, convenient, and simple processes to preserve the DNA at room temperature eliminating the dependency on cold chain systems. The card supports two-stage DNA purification for CB-NAAT and NAAT protocols, with an optional one-tube whole genome amplification for enhanced sensitivity in NAAT-like LPA.
As next steps, to fully utilize the TBSend card across the country an independent biosafety study of the TB Send Card in a real-life simulated environment is essential. It will be followed by a comprehensive multi-centric study to validate the TB Send card’s effectiveness in the tuberculosis diagnostic pathway. Post this, obtaining a manufacturing license by applying with MD-28 and receiving the license in MD-29 CDSCO form are critical steps for the TB Send card to enter the public health system
7. What role is IHF playing in the TBSend card’s journey?
IHF has been providing invaluable support to assess and implement TB Send cards. Their generous funding and unwavering commitment are driving the advancement of this unique healthcare solution, revolutionizing TB diagnosis. IHF has also facilitated collaboration with esteemed partners like ICMR-NIRT for the biosafety assessment and related studies on the TBSend Card. Their efforts in bringing together diverse expertise and resources from their internal team as well as outside agencies have been pivotal in driving the success of this project. With IHF’s support, Wobble Base is leveraging collective knowledge and capabilities to tackle the challenges of tuberculosis molecular diagnostics, specifically the “Sample to DNA” process, using the innovative TBSend card solution.
8. What is on the cards next for the TB Send card and can you identify any specific areas in diagnostics where you would like to see more innovation?
TB Send card looks forward to its universal application by bringing under its umbrella a larger spectrum of clinical samples where both DNA, as well as RNA, can be archived and subjected to both qualitative as well as quantitative detection. It sees itself as a unique nucleic acid storage device that can archive the entire nucleic acid content of a clinical sample in its near-exact form for a long period, allowing its easy and convenient storage as well as retrieval of content from time to time for all types of NAAT platforms.
About the Author:

Dr. Pratap N Mukhopadhyaya is a researcher in the field of tuberculosis diagnostics with over 20 years of experience. He has dedicated his career to advancing the understanding and detection of TB, contributing significantly to the development of cutting-edge molecular diagnostic tools. Dr. Pratap’s work has been published in numerous scientific journals and he is an inventor of several patents. Through his research and collaborations, he continues to drive innovations that improve TB diagnosis and treatment.
About Wobble Base:

Wobble Base Bioresearch Pvt Ltd. set up in 2013 is an Indian for-profit company based out of Surat, India. It is a biotechnology-driven company founded on strong core principles of advanced research and innovation. It discovers, develops and delivers novel and economic diagnostic solutions in the domain of infectious diseases and human cancer.
